Synergistic Association of Candida albicans and Streptococcus mutans in the oral cavity
By Dr.A K M Shafiul Kadir
The oral microflora is a complex niche that is conducive to the growth of a multitude of microorganisms. Several bacteria, fungi, viruses, and even protozoa reside on this surface. This plethora of microbes survives in the oral cavity due to the ample nutrients transported to the microbes via saliva. Interestingly enough, they also exhibit an astounding diversity between the species.
The diverse microbial species co-exist as biofilms on the surface of the oral cavity. Within these biofilms, the microbes interact and give rise to several inter-species or polymicrobial interactions.
Synergistic interactions occur when the presence of one microorganism favors the growth of the other organism in the same region. In contrast, if the growth of one species prevents or inhibits that of the other, then it is called an antagonistic interaction.
Our oral hygiene practices and diet have a significant impact on the diversity of the oral microbial species. Frequent sugar consumption and poor oral care lead to an increase in harmful microbes, including Streptococcus mutans (S. mutans).
Dr.A K M Shafiul Kadir collecting dental plaque biofilms using sterile cotton swab.
This bacterium has a strong relationship with another natural commensal oral cavity called Candida albicans (C. albicans). The synergistic interaction between these two species has been well-established owing to their involvement in the development of dental caries.
This article aims to thoroughly analyze this synergistic interaction and identify the clinical effects of the relationship between this bacteria and fungus.
Why are oral biofilms formed?
The teeth, tongue, cheeks, gingival sulcus, tonsils, saliva, hard palate, and soft palate offer a rich environment for these microbes to flourish. Nevertheless, their survival is not based on this alone.
Several microbes aggregate together to form "communities" by attaching themselves to the natural dentition (natural teeth) or dental prostheses such as dental implants and dentures.
The early colonizing bacterial species that directly attach to the tooth surface are often referred to as "pioneer bacteria." Moreover, this dynamic survival strategy in which dense association of the microbes occurs on the surface of the oral cavity is termed "oral biofilms."
Through biofilm formation, microbes protect themselves from the challenging environment of the oral cavity by developing specific defense mechanisms. For instance, the biofilm-forming cells of S. mutans have shown better acid tolerance response (ATR) when compared to the free-floating or planktonic cells.
Newly formed dense biofilm cells of this bacteria have shown increased resistance to the acidic condition or low pH of the environment by increasing the expression of proteins of the glycolytic pathway.
Microbial interactions within biofilms
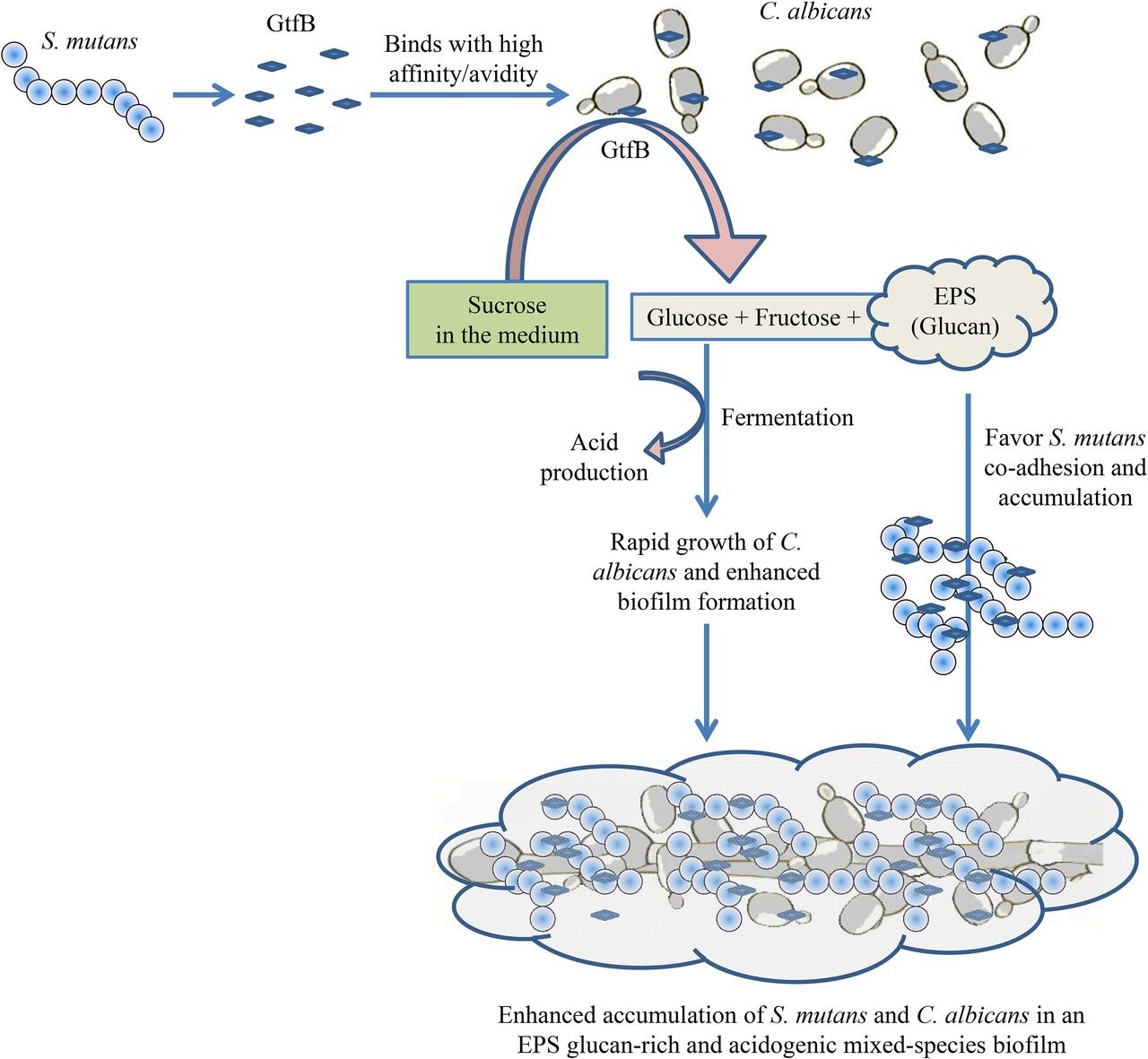
Figure collected from the article titled "Multi-omics Analyses Reveal Synergistic Carbohydrate Metabolism in Streptococcus mutans-Candida albicans MixedSpecies Biofilms" published by Amerian Society for Microbiology. https://journals.asm.org/doi/10.1128/IAI.00339-19
Although biofilms serve as a protective shield to oral microbes, they have a detrimental impact on human health. The dental plaque is a well-known example of a sophisticated oral biofilm.
The microbes residing within the dental plaque are generally symbiotic or "friendly commensals" and exist in close contact or coaggregate with each other. This proximity is beneficial in bringing about distinct cell-to-cell interactions and altering the diversity of the microbial biofilm by the release of specific signaling molecules.
Under normal circumstances, the commensals present in the oral cavity of the healthy person predominate in the biofilms. These compete with the pathogenic bacteria and inhibit their attachment to the dental surface or the pioneer bacteria.
However, when the homeostatic balance between the beneficial and pathogenic bacteria gets breached, the antagonistic effect of the friendly commensals diminishes, and the virulent species begin to flourish in the biofilms.
The virulent species are notorious for their ability to produce large amounts of metabolites (such as organic acids) which lowers the pH of the oral cavity. In addition to this, they produce complex polymers called exopolysaccharides (EPS) that prompt the adhesion of the virulent species with the tooth surface.
In this manner, they establish themselves as the main culprit in many oral diseases, such as periodontitis and dental caries.
These species also show synergistic interactions with the pioneer bacteria and promote a niche for other pathogenic bacteria.
This consortium of pathogenic species trigger the natural immune response and eventually results in the initiation and progression of several oral diseases.
Role of S. mutans and C. albicans in tooth decay
Figure of ECC collected from https://www.carltonhousedental.co.uk/all-about-tooth-decay/tooth-decay-blog/
S. mutans is a major cariogenic bacteria frequently isolated from the carious lesions of early childhood caries (ECC) and C. albicans. This bacteria breaks down carbohydrates into organic acids such as lactic acid and an insoluble sucrose-dependent EPS called glucan.
This sticky, extracellular EPS is synthesized by glucosyl transferases (GTFs) acting on the dietary sugars and forming plaque by increasing the colonization of the other species. The lactic acid produced by the metabolism of sucrose, and other sugars, such as glucose, fructose, and lactose, initiates the decay of the highly mineralized tooth enamel.
An opportunistic fungus that is commonly found in dental plaque of ECC is C. albicans. This fungal pathogen occurs in both yeast and hyphal forms.
Usually, in a healthy, caries-free individual, this species is found in tiny proportions and is therefore considered a commensal. However, excess dietary sugar consumption favors its transition to the hyphal form, which is central to the formation of biofilms and its pathogenesis.
The virulent nature of this pathogen is attributed to its ability to tolerate low pH and damage the enamel by the production of acids. It does this by actively transporting protons (H+) outside the cell through the ATPase proton pump and developing a high acid tolerance.
Moreover, it uses only glucose and fructose to secrete pyruvic acid and acetic acid, which eventually results in the rapid acidification of the local microenvironment. Furthermore, this fungus can degrade dentin under acidic conditions by releasing a protease enzyme called aspartyl protease.
Synergistic interaction of S. mutans and C. albicans
The fungal-bacteria relationship within the dental plaque is synergistic, as shown by the metabolic interactions between them. C. albicans coaggregates with S. mutans and forms a mixed-kingdom biofilm composed of a glue-like polymer of glucan.
It usually requires a carbon source for its growth, and this is provided by the lactic acid released by S. mutans. On utilizing the organic acid, the fungus lowers the oxygen tension and favors the growth of facultative streptococci by stimulating the release of growth-stimulating factors.
As well as this, the bacteria also deposits exoenzymes such as GTFs on the surface of the fungal cell wall and produce glucans in the presence of sucrose. Interestingly enough, sucrose is crucial for the fungal-bacterial interaction, as the fungus does not adhere to the bacteria or colonize the dental surfaces in its absence.
The glucans secreted by the bacteria increase the binding of the two species wherein the fungal mannans (biomolecule present in the fungal cell wall) anchor the bacteria to the mucosal surface of the oral cavity.
This also serves as a protective mechanism to the bacteria as it prevents them from being removed along with the salivary flow. In addition to this, the glucans formed during this interaction stimulate the formation of a robust mixed-species biofilm and enhance the dental plaque's virulence.
The increased microbial load in the mixed-kingdom biofilm in ECC leads to enamel dissolution in a sugar-rich environment. Tooth decay and subsequent dental caries finally manifest due to the combined acidogenic and aciduric properties of the two microbes.
The bottom line
ECC is a hyper-virulent tooth decay that rapidly and aggressively destroys smooth dental surfaces. This major health issue is often treated with surgical interventions under general anesthesia and is extremely expensive to treat.
Proper oral hygiene and limited intake of dietary sugars have some positive effects on ECC, but these cannot be used as the mainstay treatment for dental caries. Developing an effective therapy for this disease is therefore of quintessential importance.
Understanding the synergistic relationship between S. mutans and C. albicans will be an excellent asset for devising novel therapeutic approaches for ECC. These species have been involved mainly in the pathogenesis of dental caries.
This, however, requires more detailed studies of the events occurring within the mixed-kingdom biofilms to identify the targets that can be optimized for the development of effective agents.
Thus, efforts must be made to improve our knowledge on this inter-species interaction to exploit the clinical potential of this relationship in dental caries and other oral diseases.